Turning Japan’s Medical Breakthroughs into a Business
Modest might be a way of describing Cyfuse Biomedical’s office in central Tokyo, but spectacular would be appropriate to describe its funding. Its website states that in the decade since it was founded, Cyfuse’s fundraising in support of its two ground-breaking bio 3D printers brought in 540 million yen ($5.1 million) in 2013, 1.4 billion yen ($13 million) in 2015 and 1.5 billion yen ($14 million) in 2018. On top of that, Cyfuse increased its capital in 2017 by 1.1 billion yen ($10 million) through an allocation of new shares.
The attraction of Cyfuse is its innovative research into organ replacement and tissue generation. The Tokyo-based company is among a number of Japanese small and medium enterprises that are making huge technological advances in the medical field. Mitaka Kohki is another with its game-changing Medical Imaging Projection System (MIPS) for use in surgeries, while crossMedical has used its engineering prowess in a completely different way to morph from an industrial company making plastic prototypes to one that can manufacture true replicas of patients’ hearts on which doctors can simulate surgery.
Bio Printing Market Heats Up
Cyfuse has entered into business partnerships with Marubeni and Sysmex as it seeks to enhance its own platform technology through commercialization of two bio 3D printers: Regenova® and S-PIKE®. Regenova produces human tissue and organs from donor cells for clinical use, while S-PIKE produces the same for non-clinical use such as drug screening.
Cyfuse has also received assistance from two government agencies that facilitate and support research, development and business – the Japan Agency for Medical Research and Development (AMED) and the New Energy and Industrial Technology Development Organization (NEDO). Cyfuse’s Chief Financial Officer Sanjo Masahiro says that his company took advantage of government support to help deal with the difficulties and time-consuming aspects of commercialization following product development: “Fortunately, since our founding, AMED and NEDO have provided continuous support in promoting our main project. We have been able to steadily drive forward with the project thanks to their critical assistance, including regulatory advice. Also, their endorsements have been a big help as we move into unknown territory.”
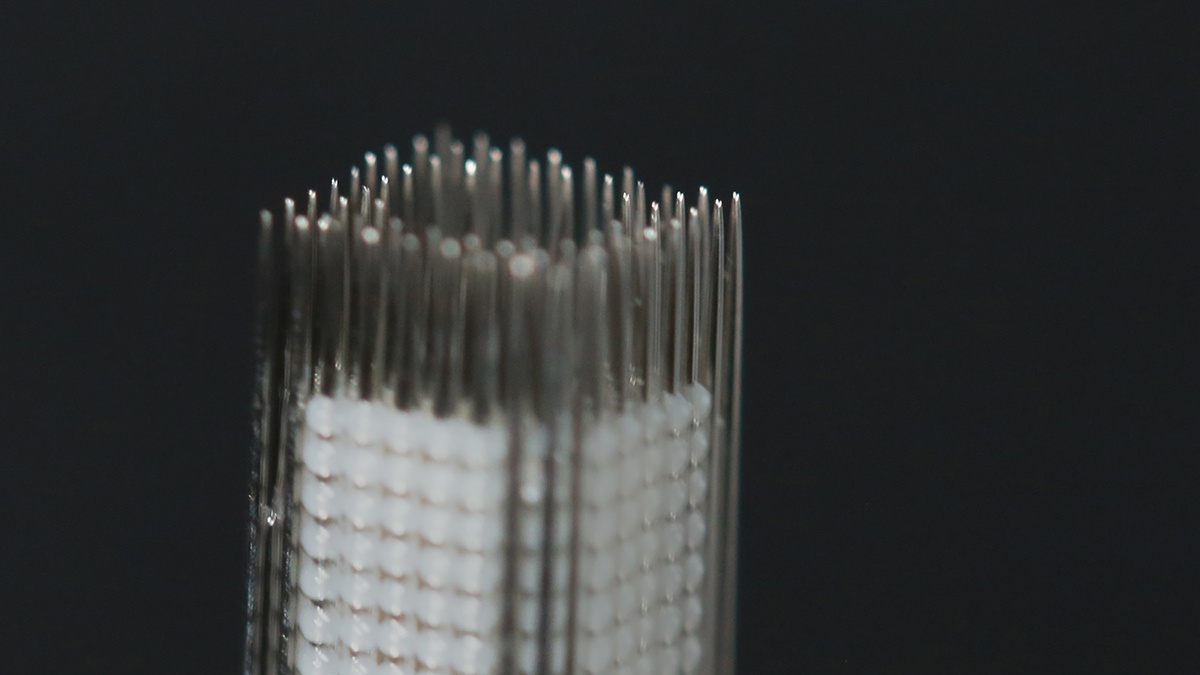
3D bio printing is still an evolving technology and not exclusive to Cyfuse or Japan. But Cyfuse’s unique kenzan method, which solved the problem of providing a temporary scaffolding on which cell spheroids can grow, has put this Japanese startup at the forefront of this technology. Regenova and S-PIKE can recreate body parts from your own cells. They are the brainchild of Professor Nakayama Koichi of Saga University who solved the critical problem of how to create a scaffold around which the new parts could be grown. His solution was a network of needles similar to the kenzan used in ikebana flower arranging.

A Game-Changer for Surgeons
Mitaka Kohki and crossMedical’s products are equally innovative and have the potential to reduce patient suffering and lower medical costs. Mitaka Kohki’s MIPS system is a game-changer for surgeons and is currently applicable to liver and breast cancer patients. To date, fluorescent imaging machines that use dye to show blood flow, have required a screen that surgeons must look at while operating. This is distracting and potentially dangerous. MIPS uses blood flow to determine the location of an incision – currently for liver or breast cancer surgery – but instead of projecting the image onto a screen, the image is projected back onto the body within 0.2 of a second and with a margin of error of 2 mm or less.
“With MIPS, doctors no longer have to look away from the area where they are doing surgery to a monitor and it provides real-time information at all times, so this is truly revolutionary,” says Seo Satoru, an Associate Professor in the Department of Surgery at Kyoto University Hospital. “Surgeries can be performed much quicker and, as a result, the trauma for patients can be mitigated and it can speed up their post-operative rehabilitation.” The product was only launched this year and is currently functioning in two hospitals.

Putting Your Heart into the Business
If you want to buy a true facsimile of crossMedical CEO Takeda Masatoshi’s heart, it will cost you around 50,000 yen ($466). It’s the company’s bestseller, mainly because these heart simulators are used for medical training. They are based on the CEO’s own heart and the company has sold around 1,500 so far. While the training hearts are useful, the simulated hearts for surgical use are life savers. Knowing crossMedical’s prototype forming business, a doctor begged Takeda to make a simulated heart so that he could visualize the surgical process required for one of his patients. The doctor told him how one in a hundred children are born with heart defects and having a simulated heart prior to surgery would save lives. With the support of AMED, crossMedical took two years to develop a system of producing near perfect replicas of patients’ hearts that could be “pre-operated” on, allowing surgeons to discover potential problems before surgery.
“Someone actually told me that prototyping means that you are going to be undertaking something that’s never been done before,” CEO Takeda says. “This means that it will be an unprecedented endeavor so you will require a very strong challenging spirit, and that interested me very much. So, of course, I decided to undertake this challenge, which had never been tried by anyone before.”

Cyfuse’s Sanjo sums up the challenge and route to success that his company faced venturing into the scientific unknown: “We started small and slowly grew into something larger. Our development reflects the nature of monozukuri [“the art of making things”], which is unique to Japan. Combining this monozukuri culture with cutting-edge technology makes us stand out as an innovative force.”
Note: All Japanese names in this advertorial are given in the traditional format, with the family name preceding the given name.
To learn more about Cyfuse Biomedical K.K., click here.
To learn more about crossMedical, Inc., click here.
To learn more about Mitaka Kohki Co., Ltd., click here.
To learn more about AMED (Japan Agency for Medical Research and Development) and its Medical Device Development Programs, click here.
Read other articles, click here.